Which Best Explains the High Surface Tension of Water
AHigh surface tension of water is best explained by its property of adhesion. Which best explains why water is able to stick to the side of glass.

Teaching Tools Surface Tension Surface Tension Tension Surface
They contain both hydrophilic groups their heads and hydrophobic groups their tails.

. At the bulk of the liquid the molecules have neighboring molecules on each side. Water has high surface tension the best way to explain this obsevation is. Cohesion is the one among the following choices given in the question that best explains the surface tension of water.
Expert answeredginabrmjPoints 8136 Log in for more information. Ahigh surface tension of water is best explained by its property of adhesion. Capillary action cohesion adhesion sublimation.
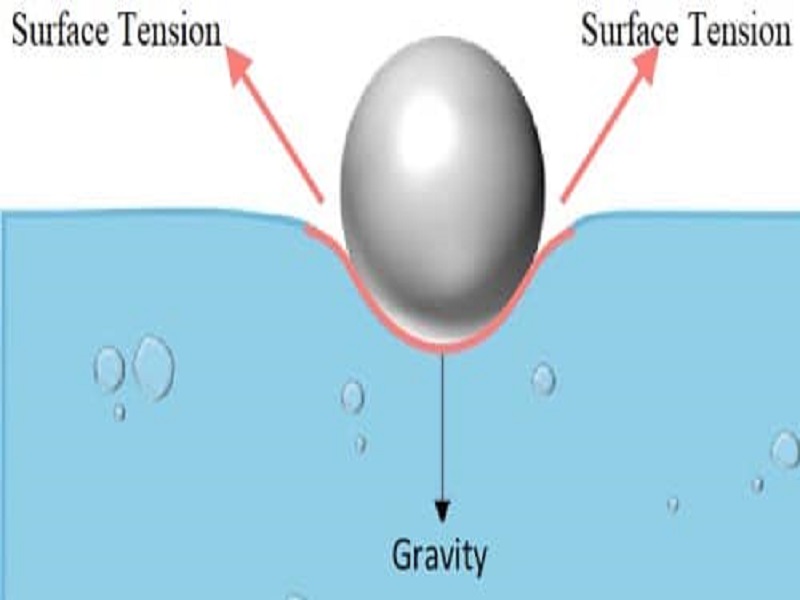
It seems to defy the laws of physics but a paper clip made of steel can indeed float on the water surface. In simple words we can say a surfactant reduces the surface tension of a liquid such as water by adsorbing at the liquid-gas interface. Which best explains the high surface tension of water.
Cohesion best explains the surface tension of water. The high surface tension helps the paper clip - with much higher density - float on the water. Its hydrogen bonding interactions.
Which best explains the surface tension of water. Surface tension in liquid water resists an external force because of cohesive nature due to hydrogen bonding of its molecules. What this means is that a surfactant has both a water-soluble.
Water molecules are polar because the. It seems to defy the laws of physics but a paper clip made of steel can indeed float on the water surface. The Urinary Bladder is filling with Urine and the Micturition Reflex.
Adhesion is the attraction of water molecules to water. Cohesion is the attraction of water molecules with other water molecules. Cohesion is the attraction of water molecules with other water molecules Eliminate.
Life As We Know It Boiling And Melting Points High Surface Tension Terms in this set 35 Water has a bent geometry. Cohesion is the attraction of water molecules with other water molecules. Which best explains the high surface tension of water.
Water molecule has a bent shape. Its hydrogen bonding interactions. On the other hand surfactants are said to be amphiphilic.
Molecules are pulling each other equally in all directions causing a net force of zero. Strong adhesive forces exist between glass and the water molecules. Which property of water best explains the high surface tension.
Water molecules are polar because the B. Water has a high surface tension. The high surface tension of water is caused by strong molecular interactions The surface tension arises due to cohesive interactions between the molecules in the liquid.
Which property of water best explains the high surface tension. Adhesion cohesion and capillary action are properties of water most closely related to Surface Tension. Water molecules on the surfa.
Which property of water best explains the high surface tension. Describe the process of Urination in the Urinary Bladder. The molecules at the surface of a glass of water do.
Blue Panda has preferred stock that pays a dividend of 700 per share and sells for 100 per share. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Which explanation does not explain why.
Some insects can stride across the water due to the combination of their legs and the high surface tension of the water. Explain how waters high heat capacity is important to living things as a temperature buffer. Cohesion is a property of water that enables the high surface tension.
Cohesion is a property of water that enables the high surface tension. Answer 1 of 2. Which best explains the high surface tension of water.
B Cohesion is a property of water that enables the high surface tension. As the other author stated the hydrogen bonding in water which are the forces holding water molecules together is extremely powerful for an intermolecular force. Adhesion is the attraction of water molecules to water molecules.
This holds the water molecules together very well causing a skin to. Asked 10222020 42121 PM. The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension.
The high surface tension of water so as many other of its properties is due to the strong attraction force created by the hydrogen bonding which is the attraction between neighbors hydrogen atoms with a partial positive charge and oxygen atoms with partial negative charge Answer is its hydrogen bonding. Electrons are evenly distributed in the water molecule. Its hydrogen bonding interactions.
The high surface tension helps the paper clip - with much higher density - float on the water. Explain why waters high heat of vaporization is important to living things as. Water has several unique properties such as high boiling point high surface tension and low vapor pressure The type of attraction that best accounts for these unique properties is.

Is7012 Surface Tension Of Water Surface Tension Next Generation Science Standards Tension

Surface Tension What Is It How Does It Form What Properties Does It Impart Youtube

10 Surface Tension Examples In Daily Life Studiousguy

Surface Tension Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
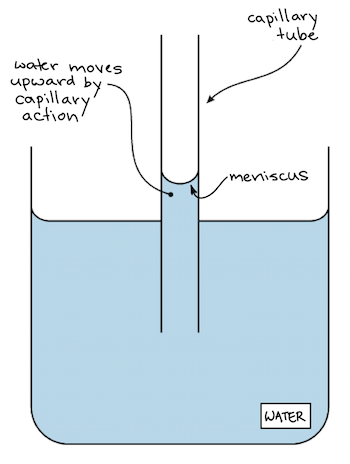
Cohesion And Adhesion Of Water Article Khan Academy

Surface Active Agents Surfactants Types And Applications

Hydrogen Bonds Make Water Sticky Manoa Hawaii Edu Exploringourfluidearth
10 Surface Tension Examples In Daily Life Studiousguy

Surface Tension Allows A Water Strider To Walk On Water U S Geological Survey

Surface Tension Chapter 5 The Water Molecule And Dissolving Middle School Chemistry

3 Ways To Measure Surface Tension
Water Striders National Wildlife Federation

Use Surface Tension To Make Pepper Dance Scientific American

What Is Surface Tension Definition And Causes Surface Tension Tension Surface

7 Science Tricks With Surface Tension Youtube

Surface Tension Chapter 5 The Water Molecule And Dissolving Middle School Chemistry

Cohesion And Adhesion Of Water Article Khan Academy
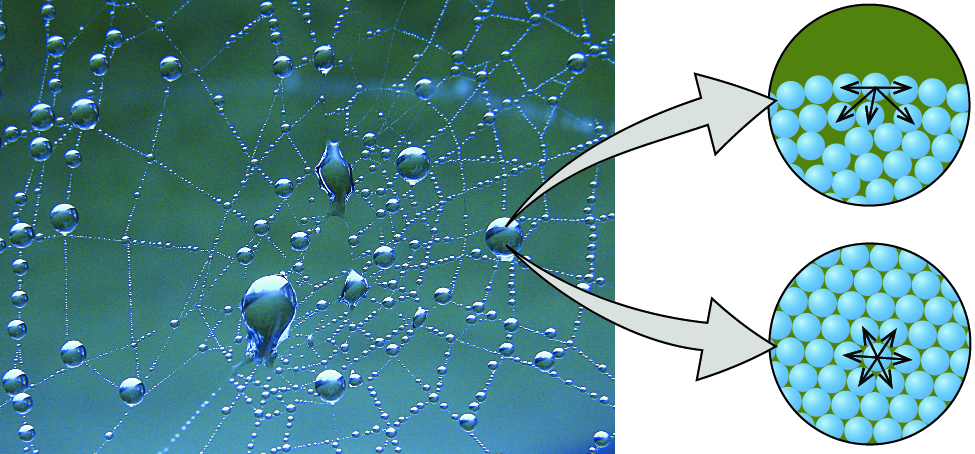
Cohesion And Adhesion Of Water Article Khan Academy

Paper Clip Can Float On Water Due To High Surface Tension Of Water U S Geological Survey
Comments
Post a Comment